Formulas Of Binary Ionic Compounds
7.viii: Formulas for Binary Ionic Compounds
- Page ID
- 53722
How does autograph work?
Shorthand was a very popular way of recording speech, especially in dictating letters and in court testimony. Instead of trying to write out all the words, the person taking the dictation would use a ready of symbols that represented syllables or words. The pages above show a autograph version of "A Christmas Carol" written past Charles Dickens. Unless you know shorthand, the passage is meaningless. But knowing shorthand allows you to read this classic story. Different professions also use a type of shorthand in communication to save fourth dimension. Chemists use chemical symbols in combination to indicate specific compounds. There are two advantages to this approach:
- The compound nether discussion is clearly described then that there can be no confusion about its identity.
- Chemical symbols represent a universal language that all chemists can empathize, no matter what their native language is.
Writing Formulas for Binary Ionic Compounds
If you know the name of a binary ionic compound, you can write its chemic formula. Start by writing the metallic ion with its accuse, followed by the nonmetal ion with its charge. Because the overall compound must be electrically neutral, determine how many of each ion is needed in social club for the positive and negative accuse to cancel each other out. Consider the chemical compound aluminum nitride. The ions are:
\[\ce{Al^{3+}} \: \: \: \: \: \ce{Northward^{iii-}}\nonumber \]
Since the ions take charges that are equal in magnitude, ane of each will be the lowest ratio of ions in the formula. The formula for aluminum nitride is \(\ce{AlN}\).
The ions for the chemical compound lithium oxide are:
\[\ce{Li^+} \: \: \: \: \: \ce{O^{2-}}\nonumber \]
In this example, two lithium ions are required to balance out the charge of one oxide ion. The formula of lithium oxide is \(\ce{Li_2O}\).
An alternative way to write a correct formula for an ionic chemical compound is to apply the crisscross method. In this method, the numerical value of each of the ion charges is crossed over to become the subscript of the other ion. Signs of the charges are dropped. Shown below is the crisscross method for aluminum oxide.
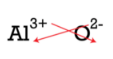
The red arrows bespeak that the 3 from the \(iii+\) accuse will cantankerous over to get the subscript of the \(\ce{O}\). The 2 from the \(2-\) charge will cross over to go the subscript of the \(\ce{Al}\). The formula for aluminum oxide is \(\ce{Al_2O_3}\).
Be aware that ionic compounds are empirical formulas then must exist written as the lowest ratio of the ions. In the instance of aluminum nitride, the crisscross method would yield a formula of \(\ce{Al_3N_3}\), which is not right. It must be reduced to \(\ce{AlN}\). Post-obit the crisscross method to write the formula for lead (Iv) oxide would involve the following steps:
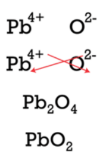
The crisscross first yields \(\ce{Pb_2O_4}\) for the formula, but that must be reduced to the lower ratio, and \(\ce{PbO_2}\) is the correct formula.
Summary
- Formulas for binary compounds begin with the metal followed by the nonmetal.
- Positive and negative charges must cancel each other out.
- Ionic compound formulas are written using the everyman ratio of ions.
Review
- Write formulas for the binary ionic compounds formed between the following pairs of elements:
- cesium and fluorine
- calcium and sulfur
- aluminum and chlorine
- zinc and nitrogen
- Write the formula and give the name for the compound formed by the following ions:
- Atomic number 263 + and O2-
- Ni2 + and Southii-
- Au+ and Cl-
- Sn4 + and I-
- Give names for the following compounds:
- AgtwoDue south
- PdO
- PtCliv
- ViiOfive
Formulas Of Binary Ionic Compounds,
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/07%3A_Chemical_Nomenclature/7.08%3A_Formulas_for_Binary_Ionic_Compounds
Posted by: lightliess1983.blogspot.com


0 Response to "Formulas Of Binary Ionic Compounds"
Post a Comment